On the 4th January 2021, 82-year-old Brian Pinker became the first person to receive the Oxford-AstraZeneca vaccine which was approved by the MHRA for UK distribution on the 30th December 2020.[1] This follows the MHRA’s approval of the Pfizer-BioNTech vaccine on the 2nd December 2020[2]; inoculation using this vaccine began on the 8th December 2020 with Margaret Keenan becoming the first-ever recipient.[3] However, transporting, storing and distributing injections brings its own set of logistical problems as their viability is dependent on maintaining specific temperatures which vary from vaccine to vaccine. Monitoring and maintaining temperature is therefore essential to ensuring successful mass inoculation. Many companies, such as Comark, Testo and FilesThruTheAir, have developed temperature data logging solutions optimised for this purpose.
Quick Links
Vaccine Overview
Oxford-AstraZeneca
The Oxford-AstraZeneca injection is a viral vector (a genetically modified virus) vaccine.[4] As a result, it does not have to be frozen unlike the Pfizer-BioNTech and Moderna vaccines. Like most injections, the Oxford-AstraZeneca jab can be stored, transported and handled at 2°C-8°C, the standard temperature range of a fridge. It can be kept in a vaccine refrigerator for at least six months.[5] This should aid in the rollout of the vaccine as additional provisions to maintain extra-low temperatures do not have to be made.
The UK has purchased 100 million doses of this vaccine, enough to inoculate 50 million people (if everyone receives 2 doses), at a cost of approximately £3 per shot. Phase three testing indicates a 62-90% effectiveness.[6]
Moderna
The Moderna vaccine is an RNA-based (part of a virus' genetic code) injection. Due to this, it must be frozen to prevent degradation. The Moderna vaccine has to be transported and stored at -20°C; while this presents more of a logistical issue than the Oxford-AstraZeneca jab, it does not present the same problems as the Pfizer-BioNTech injection as it can be kept in a standard vaccine freezer. When frozen at this temperature the Moderna vaccine can be stored for approximately six months. [7]
Furthermore, when thawed the Moderna jab remains stable for longer than the Pfizer-BioNTech shot. It can be stored at refrigeration temperatures (2-8°C) for up to 30 days, and, in a worst-case scenario, will survive being left at room temperature for a maximum of 12 hours.[8]
The Moderna vaccine was approved for use in the UK by the healthcare regulator MHRA on the 8th January 2021 with the first doses expected to be administered in the spring of 2021. It is thought to have an efficacy of 94% based on preliminary phase-three results. The UK has purchased 17 million doses at £25 per dose, enough to vaccinate approximately 8.5 million people.[9] However, despite easier, more cost-effective distribution, as the Moderna jab contains 100 micrograms of mRNA per dose compared to Pfizer-BioNTech’s 30 micrograms, it may be harder to produce in large quantities.[10]
Pfizer-BioNTech
The Pfizer-BioNTech jab was the first vaccine to be approved by the MHRA for use in the UK. It has an effectiveness of 95% based on preliminary phase three results and has already begun to be administered in the UK despite relatively-complex temperature requirements.[11]
Like the Moderna vaccine, the Pfizer-BioNtech injection is an RNA-type vaccine. It contains 30 micrograms of mRNA per dose. The UK has purchased 40 million doses of the Pfizer-BioNTech jab at £15 per dose; this should be enough to inoculate approximately 20 million people.[12]
As the Pfizer-BioNTech jab needs to be stored at -70°C this presents challenges to distribution, especially as this vaccine has to be imported from Pfizer’s centres in the US, Germany and Belgium. Most GPs do not have the facilities to store vaccines at -70°C, therefore, they are being kept at ‘freezer farms’ where doses can be stored for up to six months. Pfizer-BioNTech vaccines are being transported to ‘freezer farms’, and from these farms to NHS vaccination sites, in specially designed freezer boxes packed with dry ice. These boxes can preserve the vaccine for 10 days if unopened and 30 days if the dry ice is replenished every 5 days. Once the shots have arrived at the NHS vaccination site, they are thawed and kept in a refrigerator (2-8°C) for a maximum of 3.5 days. The defrosted vaccine must be diluted in saline before being administered to a patient. It has to be used within six hours of dilution.[13]
How Can Temperature Data Loggers Help?
Temperature data loggers offer an easy and ergonomic method of measuring and recording temperatures of containers (like the aforementioned freezer boxes), vaccine refrigerators and freezers over a prolonged period of time, for example during transit and storage. This means medical professionals can ensure vaccines are kept at optimal temperatures throughout the logistical process and that degradation as a result of high temperatures has not occurred. Moreover, healthcare workers will be able to spot potential rises in temperature earlier allowing them to take corrective action sooner. This will help to prevent damage to vaccines which may render them unusable, ensuring that injections are not wasted and mass inoculation can be carried out as effectively, efficiently and reliably as possible.
Comark
Comark advises that the following temperature data loggers are suitable for monitoring vaccine storage:
- Comark RF314DUAL Diligence Wi-Fi Dual Channel Temperature Data Logger
- Comark RF516 Wireless Temperature Transmitter
- Comark N2014 Temperature Data Logger Starter Kit
Comark’s Diligence models measure the ambient temperature and can be programmed to log data to the user’s PC or to the Comark Cloud, thereby providing unlimited data recording. From these locations data may be viewed, analysed and exported into a graph, providing a comprehensive history of the fridge’s, freezer’s or transport box’s temperature history. The logging rate can be configured as necessary.
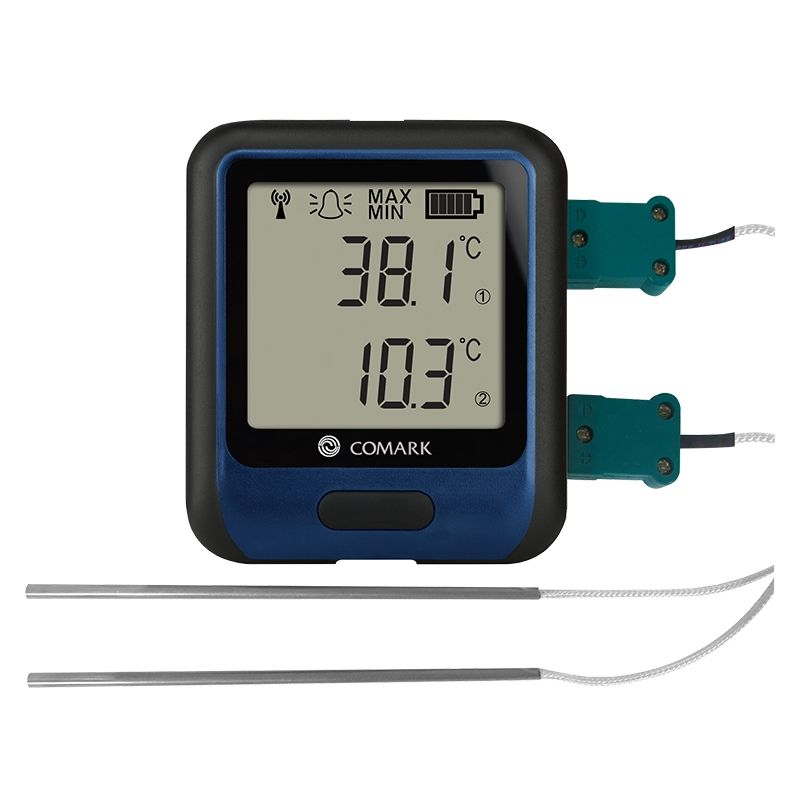
The Comark RF314DUAL Diligence Wi-Fi Dual Channel Temperature Data Logger, which measures temperatures between -270°C and +1300°C as standard, can monitor the temperature of fridges and freezers used to store all three types of the COVID-19 vaccine. This dual-channel temperature data logger also supports high and low alarms which will alert the user when temperatures exceed programmed limits, allowing medical professionals to take corrective action quickly. Data can be viewed via the LCD display.
Click here to view Comark's COVID-19 Vaccine Monitoring Support Brochure.
Testo
Testo offers a number of ranges suitable for ambient temperature monitoring; these include:
- Testo 184 Temperature Data Loggers
- Testo 174 Temperature Data Loggers
- Testo 175 Temperature Data Loggers
- Testo 176 Temperature Data Loggers
Testo advises that their 184 Series of Temperature Data Loggers are specifically designed for cold chain logistics making them perfect for monitoring the temperature of boxes and containers used to transport the Pfizer-BioNTech (Testo 184 T4 only), Moderna and Oxford-AstraZeneca vaccines. These battery-operated USB temperature data loggers support temperature ranges in the region of -80°C to +70°C (model dependent). Data is indicated on the Testo 184 Temperature Data Loggers’ displays (except the Testo 184 T1 and Testo 184 T4) and all models feature an alarm LED which clearly indicates when permissible temperatures have been exceeded.
The Testo 184 Temperature Data Loggers can be programmed to record measurements at rates ranging from every minute to every twenty-four hours. Capacity to store data varies between models from approximately 16 000 readings (Testo 184 T1) to 64 000 readings (Testo 184 H1 and Testo 184 G1). A USB design means the Testo 184 models can be plugged into a PC, allowing users to view the complete temperature history of the vaccine storage box, fridge or freezer quickly and easily.
Additionally, the Testo 184 HI and Testo 184 G1 support use with an Android smartphone, enabling medical staff to view data remotely. As a result, they may spot anomalies sooner and can take corrective action before significant damage has occurred.
FilesThruTheAir
FilesThruTheAir has developed a series of temperature data loggers specifically optimised for monitoring vaccines:
- FilesThruTheAir EL-USB-VAC Vaccine Monitoring Temperature Data Logger
- FilesThruTheAir EL-SMS-2G-VAC Vaccine Temperature Monitor with SMS Alerts
- FilesThruTheAir EL-WIFI-VAC/2 Vaccine Monitoring Data Loggers
- FilesThruTheAir EL-WiFi-VACX Vaccine Temperature Data Logger
- FilesThruTheAir EL-GFX-VAC2 2-Channel Vaccine Monitoring Data Logger
All these models feature a glycol bottle with a thermistor probe designed to mimic the temperature of vaccines which usually lags behind the temperature of the refrigerator or freezer. This means healthcare professionals are provided with a more accurate indication of the vaccine’s internal temperature. FilesThruTheAir’s vaccine monitoring data loggers can measure temperatures between -40°C and +60°C. This means they are suitable for monitoring the temperature of the Oxford-AstraZeneca and Moderna vaccines but are unable to measure temperatures low enough to store the Pfizer-BioNTech injection.
Like the Testo models and Comark’s RF314DUAL, FilesThruTheAir’s Vaccine Temperature Data Loggers feature configurable alarms and logging rates. The FilesThruTheAir EL-WIFI-VAC/2 Vaccine Monitoring Data Loggers alert the specified user via email when an alarm has been triggered. Similarly, the FilesThruTheAir EL-SMS-2G-VAC Vaccine Temperature Monitor with SMS Alerts will send a text message to the user-designated number every time the alarm limits are breached; this data logger can also be programmed to send a daily, weekly or monthly summary text providing individuals with an overview of the fridge’s or freezer’s temperature history for that time period.
The FilesThruTheAir EL-WIFI-VAC/2, EL-WiFi-VACX and EL-GFX-VAC2 Vaccine Monitoring Data Loggers can be set to record data to a PC or the EasyLog Cloud. However, the FilesThruTheAir EL-USB-VAC Vaccine Monitoring Temperature Data Logger saves up to 32 000 measurements to its internal memory; stored data may be downloaded to a PC by plugging the data logger into a USB port.
Further Information
For more information regarding any of the aforementioned temperature data loggers please contact our sales team on 01642 931 329 or via our online form.
Alternatively, please visit our dedicated category for Cold Storage & Vaccine Temperature Dataloggers.
[1] Hannah Boland, Annelies Gartner, Christopher Hope and Lizzie Roberts, ‘Covid-19 vaccine: Latest Updates on Oxford, Moderna and Pfizer breakthroughs – and who will get it first’, The Telegraph, last accessed 08 January 2021.
[2]David Cyranoski, Heidi Ledford and Richard Van Noorden, ‘The UK has approved a COVID vaccine – here’s what scientists now want to know’, Nature, last accessed 08 January 2021.
[3] Boland, Gartner, Hope and Roberts, ‘Covid-19 vaccine: Latest Updates on Oxford, Moderna and Pfizer breakthroughs – and who will get it first’
[4] Dominic Bailey and Lucy Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’, BBC, last accessed 08 January 2021.
[5] AstraZeneca, AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK, last accessed 08 January 2021.
[6] Bailey and Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’
[7] Bailey and Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’
[8] Lucia Binding, Alix Culbertson and Alexa Phillips, ‘COVID-19 vaccines: How do the Moderna, Pfizer and Oxford coronavirus jab candidates compare?’, Sky News, last accessed 08 January 2021.
[9] Michelle Roberts, 'Covid vaccine: Moderna seeks approval in US and Europe' and 'Moderna becomes third Covid vaccine approved in the UK', BBC, both last accessed 08 January 2021.
[10] Binding, Culbertson and Phillips, ‘COVID-19 vaccines: How do the Moderna, Pfizer and Oxford coronavirus jab candidates compare?’
[11] Bailey and Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’
[12] Bailey and Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’
[13] Bailey and Rodgers, ‘Covid vaccine: How will the UK jab millions of people?’